Introdution
Glioma is a central nervous system tumor disease, derived from glial cell lesions. It is one of the most common primary brain tumors, accounting for 35-60% of intracranial tumors, and the adult incidence rate is as high as 8-10/100,000, the 5-year survival rate is 20%-30%.Next, we will introduce the pathogeny,classification, location, pathological classification and molecular classification of glioma.
Grade
The WHO divides gliomas into grades 1 to 4 according to the degree of malignancy,
and grades 1 to 2 are low-grade gliomas, which are well-differentiated gliomas;
although this type of tumor is not biologically benign,
it is The prognosis of the patient is relatively good.
Grades 3 to 4 are high-grade gliomas, which are poorly differentiated gliomas.
Such tumors are malignant tumors and the prognosis of patients is poor.
Classfication
According to the histological and molecular pathological characteristics, gliomas are divided into five groups:adult diffuse gliomas;Children's diffuse low-grade glioma;Children's diffuse high-grade glioma;Localized astrocytoma;Epithelial tumors.
Among them, adult diffuse gliomas are further divided into three types based on histology, mutation status of isocitrate dehydrogenase (IDH), and several other key molecular characteristics:
1.IDH mutant astrocytoma is classified as WHO grade 2, 3, or 4 based on histological features such as nuclear atypia, mitosis, endothelial proliferation, necrosis, and molecular changes.
2.IDH mutant, 1p/19q co deletion oligodendroglioma is a diffuse glioma defined by both histological and molecular characteristics, characterized by the presence of both IDH1/2 mutation and chromosome arm 1p/19q co deletion. Based on histological characteristics. It is also classified as WHO2 or Level 3.
3.IDH wild-type glioblastoma is the most common malignant tumor among adult primary brain tumors.
Citation:
Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY.Primary brain tumours in adults.Lancet.2012.379(9830):1984-96
Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D,Cavenee WK, Ohgaki HWiestler OD,Kleihues P,EllisonDW: The 2016 World Health Organization Classification of Tumorsof the Central Nervous System: a summary. Acta Neuropathol 2016,131(6):803-820.
TCR (T cell receptor) is a molecular structure that T cells specifically recognize and bind antigen peptide MHC molecules. It usually exists on the surface of T cells in the form of a complex with CD3 molecules.
Classification
There are three different T cell receptors in vertebrates, αβTCR, γδTCR and pre-TCR.
Pre-TCR is only expressed on the surface of mature αβT cells, αβTCR is expressed on the surface of mature αβT cells and NKT cells, and γδTCR is expressed on γδT cells surface.
The αβTCR on the surface of T cells recognizes the polypeptide antigen presented by MHC;
the αβTCR on the surface of NKT cells recognizes the lipid antigen presented by CD1.
T cell receptors are composed of two different subunits, 95% of T cell receptors are composed of α subunits and β subunits,
and another 5% of T cell receptors are composed of γ subunits and δ subunits.
This ratio will change due to individual development or disease.
The specific binding of T cell receptors to the polypeptides presented by MHC triggers a series of biochemical reactions,
and activates T cells through numerous accessory receptors, enzymes and transcription factors to promote their division and differentiation.
Structure
The T cell receptor is a heterodimer fixed on the cell membrane,
most of which are composed of highly variable α subunits and β subunits connected by disulfide bonds.
This type of T cell is called αβ T cell. The few containing γ and δ subunits are called γδ T cells.
The T cell receptor and the constant CD3 molecule form a T cell receptor complexus.
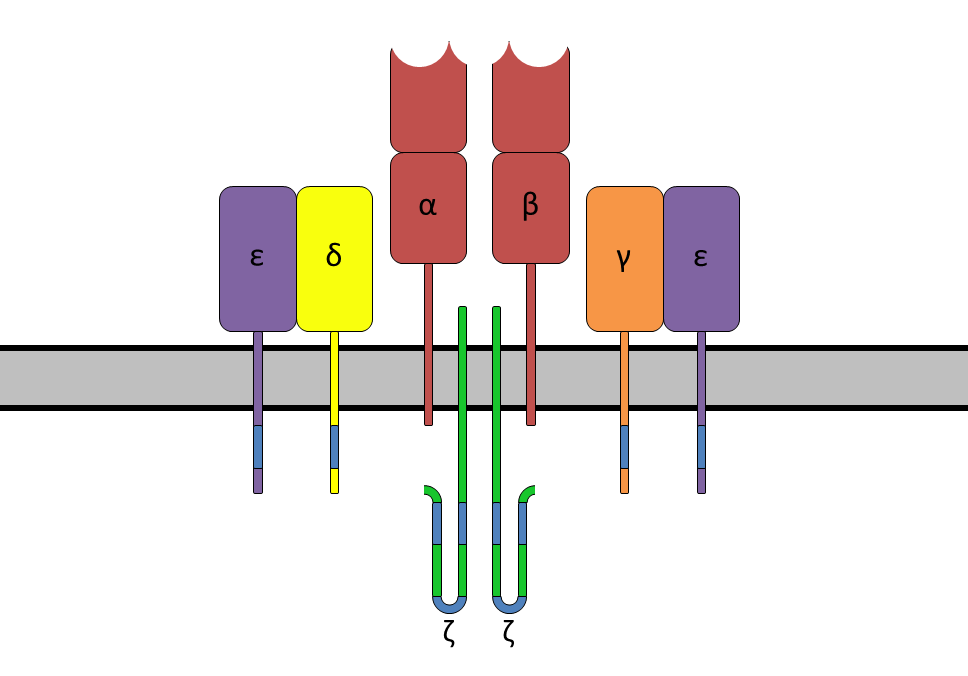
Each subunit contains two extracellular domains: variable region and constant region.
These domains belong to the immunoglobulin superfamily and are composed of anti-parallel β sheets.
The constant region is close to the cell membrane, connecting the transmembrane region and the end of the cell,
while the variable region is responsible for recognizing the polypeptide/MHC complexus.
The variable region of each subunit contains three highly variable complementarity determining regions (CDR).
The most important CDR3 is responsible for directly binding to the polypeptide presented by MHC.
The CDR1 of α subunit and β subunit respectively act on the N-terminus and C-terminus of the polypeptide.
CDR2 is believed to be involved in the recognition of MHC. The β subunit also has an additional CDR4,
which is usually not involved in the recognition of the polypeptide/MHC complex,
but is related to the role of the superantigen.
Variety
The function of TCR is to combine with antigens in the body. Due to the diversity of antigens, TCR also has great diversity in the body. There are 10^13 different TCR in normal humans. The mechanism that produces this diversity is the VDJ recombination of immunoglobulin genes. The gene locus encoding immunoglobulin is composed of many gene segments, including variable segments (V), connecting segments (J), and multiple segments (D) that may exist between them. The α and γ subunits are produced by VJ recombination, and the β and δ subunits are produced by VDJ recombination. The random recombination between different gene fragments and the randomly inserted nucleotides during the recombination process greatly enrich the diversity of T cell receptors. The figure on the right is a schematic diagram of the VDJ gene rearrangement of the β chain.
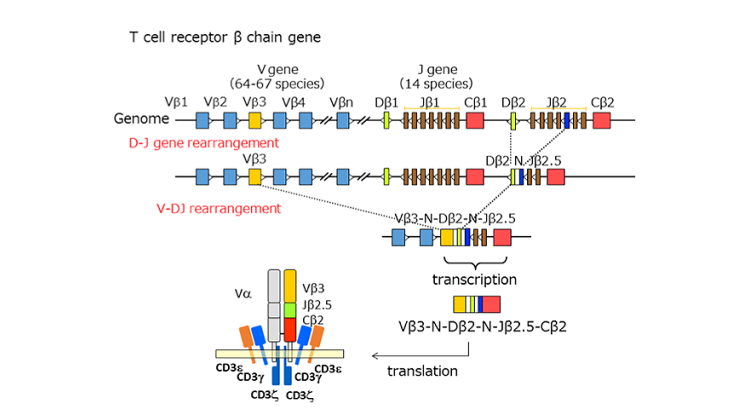
Citation:
Chen, Kou Zhong , et al. "Combination antiretroviral therapy results in a rapid increase in T cell receptor variable region beta repertoire diversity within CD45RA CD8 T cells in human immunodeficiency virus-infected children. " Journal of Infectious Diseases 3:385.379(9830):1984-96
B-cell antigen receptor (BCR) is a molecule responsible for specific recognition and antigen binding on the surface of B cells. Its essence is a membrane immunoglobulin (MIG). BCR has antigen binding specificity. The BCR diversity of each individual is as high as 5x10 ^ 13, forming a large BCR library, which gives individuals great potential to recognize various antigens and produce specific antibodies. On the surface of mature B cells, BCR always interacts with Ig, which is responsible for transmitting antigen stimulation signals α/ Ig β (CD79a / b) heterodimers are co expressed to form BCR Ig α/ Ig β Complex (BCR complex).
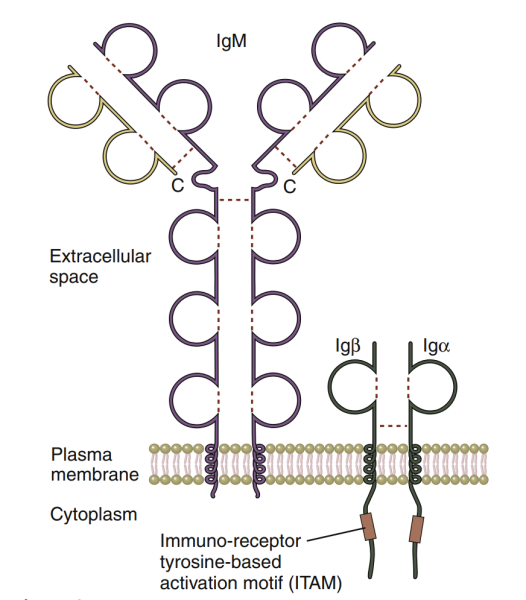
Structure
Two types of membrane immunoglobulin (MIG) are expressed on the surface of B cells: migm and MIGD, which are used to specifically recognize and bind antigens. MIG is composed of two heavy chains and two light chains. The heavy chain is divided into variable region (V region, about 110 amino acid residues), constant region (C region, about 330 amino acid residues), transmembrane region (26 amino acid residues) and cytoplasmic region (3 amino acid residues); The light chain has only V and C regions. The V region of heavy chain and light chain each has three regions with highly variable amino acid composition and arrangement order. These regions can form complementary spatial conformations with antigen epitopes, which is called complementarity determining region (CDR). The three CDRs on MIG are involved in antigenic recognition and jointly determine the antigen specificity of BCR.During the development of B cells, the expression of MIG will change: immature B cells only express migm; Mature B cells can express both migm and mlgd, which is called initial B cells; However, the MIGD on the surface of B cells gradually disappeared after activation; When B cells finally differentiated into plasma cells, they did not express MIG.
Citation:
曹雪涛.医学免疫学(第6版).北京.人民卫生出版社.2013.76-82,110-112